Regulatory Services > Registration

SME Application
Applying for EU SME (Small & Medium Enterprise) Status
Do you qualify as an SME?
The definition of an SME enterprise can be found in Article 2 of the Annex to the
*Commission Recommendation of 6 May 2003 concerning the definition of micro, small and medium-sized enterprises (2003/361/EC)
There are three main criteria used to determine SME status
- Staff headcount
- Financial turnover and balance sheet
- Ownership structure
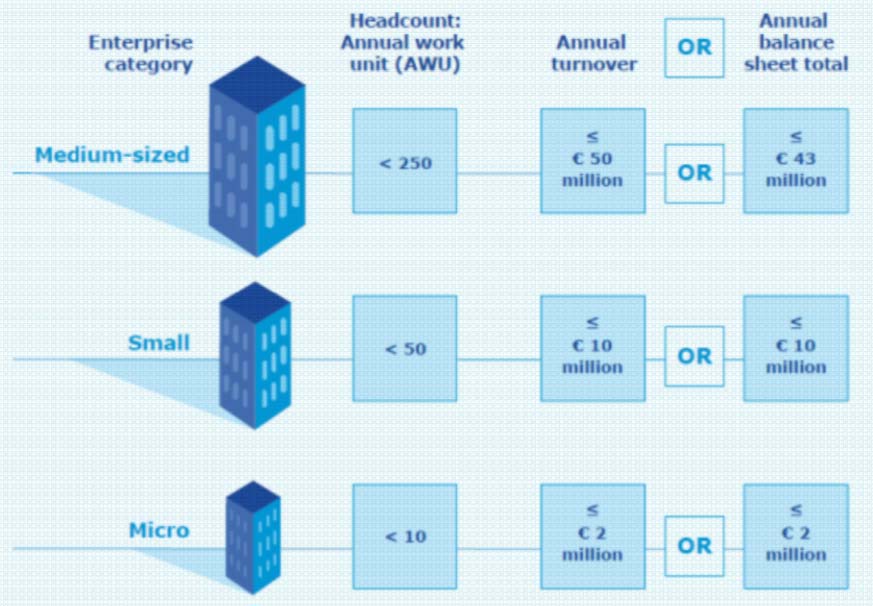
To qualify the enterprise must be in the EEA and have fewer than 250 employees and; either an annual turnover of not more than €50 million or an annual balance-sheet total of not more than €43 million.
- Within the SME category, a small enterprise is defined as an enterprise which employs fewer than 50 persons and whose annual turnover and/or annual balance sheet total does not exceed EUR 10 million.
- Within the SME category, a microenterprise is defined as an enterprise which employs fewer than 10 persons and whose annual turnover and/or annual balance sheet total does not exceed EUR 2 million.
As the ownership structure may affect the overall headcount and financial criteria it is very important to calculate the effects of any partnership or linkage with other legal entities. This is a complex area which CambReg can help you with
SME Thresholds Commission Rcommendation 2003/361/EC
Are you aware of the advantages of having SME Status?
The European Medicines Agency (EMA) provides incentives for SMEs that are developing human or veterinary medicinal products. The regulation that supports these incentives is Commission Regulation (EC) No 2049/2005, the aim of which is to promote innovation.
Once SME status is granted you can take advantage of administrative, regulatory and financial support provided by EMA, including:
- Regulatory assistance
- SME briefing meeting (free of charge) provides a platform for early dialogue with EMA to discuss regulatory strategy, product development and the range of procedures and incentives available.
- Fee exemptions and reductions e.g. Scientific Advice 90% reductions
- Assistance with translations of the product information (no cost to the applicant)
- Training and advice via Information days, News Letters and SME User Guide
- Help with partnering and networking
What can CambReg do for you?
We can assist you with your own SME application and registration process or you can take advantage of our own status as an SME
We hold SME status for both our UK based company, Cambridge Regulatory Services Ltd and our sister company based in Cyprus, CambPharma Solutions (CY) Ltd
Whilst UK is still part of the EU CambReg’s SME status remains valid, after UKs departure from the EU we can continue to offer non-EEA companies (or EU based companies that prefer to work through an already existing SME) an SME platform from which to operate