Regulatory Services > Registration

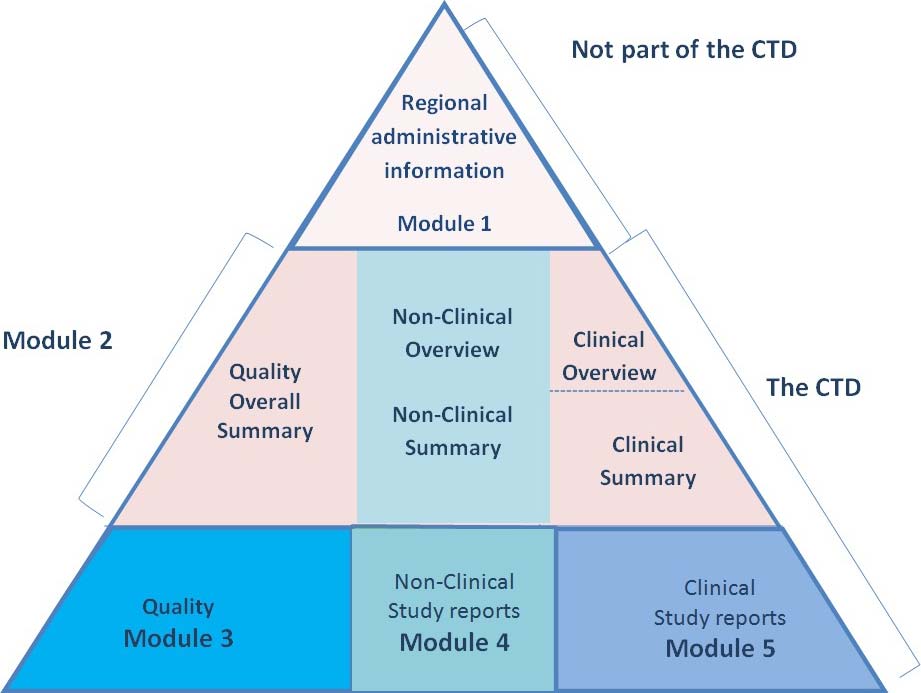
Modules 1-5 (CTD)
Common Technical Document (CTD)
To be able to sell a medicinal product in the UK/European Union a Marketing Authorisation (MA) must be obtained for each product. The Marketing Authorisation certifies that the product meets the required standards of quality, safety and efficacy.
To obtain an MA a Regulatory Dossier must be compiled, containing all relevant data relating to quality, safety and efficacy, in the form a Common Technical Document (CTD). With the exception of Module 1, which is regions specific, a CTD (Modules 2 to 5) can be submitted and accepted by any Health Authority in the EU, USA and Japan as a minimum.
Diagrammatic Representation of a CTD
CTD preparation is one of CambReg’s core competencies. As well as compilation of the data in to the correct format we can author all of the individual CTD components, including but not limited to:
- Quality overall summaries.
- Non-clinical overviews and summaries
- Clinical overviews and summaries